Abstract
Background: Treated secondary acute myeloid leukemia (ts-AML), defined as AML arising from previously treated antecedent hematologic disorder, is a high-risk disease with dismal outcomes. CPX-351 is approved for the treatment of secondary AML, although it did not improve outcomes within the subgroup of patients (pts) with secondary AML with prior exposure to hypomethylating agents (HMAs). In clinical practice, either chemotherapy or HMA plus venetoclax (Ven) are often used for pts with ts-AML, although there are little data to guide the optimal therapy in this setting
Methods: We conducted a retrospective analysis of pts with AML arising from either MDS or CMML who received first AML therapy at our institution from 6/2004 to 1/2021 and who had prior HMA exposure for preceding MDS/CMML. Pts treated with prior intensive chemotherapy (IC) or Ven were excluded. Treatment regimens were classified as IC, low-intensity chemotherapy (LIC), or HMA plus Ven.
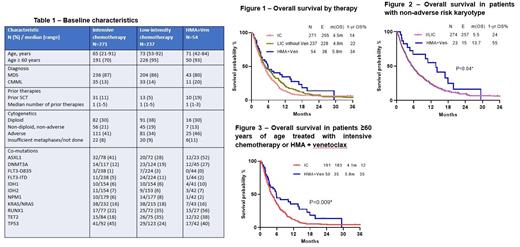
Results: Among 562 pts with ts-AML, 271 (47%) were treated with IC, 237 (41%) with LIC, 54 (9%) with HMA+Ven. Baseline characteristics were similar between pts treated with different treatment regimens (Table 1), although pts treated with IC were significantly younger than those treated with LIC or HMA+Ven (median age: 65 vs. 72 years, P<0.0001). The median number of prior therapies for previous MDS/CMML diagnosis was 1 (range, 1-5). The entire cohort was enriched with adverse risk mutations, including ASXL1 (IC-41%, LIC-28%, HMA+Ven-52%), RUNX1 (IC-22%, LIC-35%, HMA+Ven-56%), and TP53 (IC-45%, LIC-24%, HMA+Ven-40%).
In the entire cohort, the CR/CRi rate was 26% and the overall response rate (ORR; defined as CR+CRi+MLFS) was 34%. CR/CRi rates and ORR were similar between IC and LIC (CR/CRi rates: 24% vs 26%; ORR: 30% vs 35%, respectively). Compared with IC/LIC, treatment with HMA+Ven was associated with higher CR/CRi rates (39% vs 25%; P=0.03) and ORR (54% vs 33%; P=0.002). 60-day mortality was similar between the treatment groups (IC-14%, LIC-9%, HMA+Ven-13%). Hematopoietic stem cell transplant rates for pts who received IC, LIC and HMA+Ven were 13%, 5%, and 9%, respectively.
In the entire cohort, the median overall survival (OS) was 4.8 months (m), and relapse-free survival (RFS) was 5.9m. OS was similar between those treated with IC or LIC (median OS 4.5m vs. 4.8m; 1-year [yr] OS 14% vs. 22%; P=0.16) but was superior for those who received HMA+Ven compared with those who received IC/LIC (median OS 5.8m vs. 4.7m; 1-yr OS 35% vs. 17%; P=0.05 (Figure 1). RFS was also numerically higher in pts who received HMA+Ven compared with those treated with IC/LIC (median RFS 14.4m vs. 5.4m; 1-yr RFS 59% vs. 29%; P=0.17).
The impact of therapies on outcomes in ts-AML was influenced by karyotype. In those with adverse risk karyotype, the outcomes were dismal regardless of treatment approach. ORR was similar between IC/LIC and HMA+Ven (24% vs 28%, P=0.66) and these groups also had similar OS (median OS: 3.3m vs. 4.1m; 1-yr OS 8% vs. 11%; P=0.41). However, among those with non-adverse risk karyotype, HMA+Ven was associated with significantly higher CR/CRi rates (57% vs 30%; P=0.008) and ORR (78% vs 39%, P=0.0003) compared with IC/LIC. HMA+ven also resulted in superior OS in this non-adverse risk group (13.7m vs 5.5m, 1-yr OS 24% vs. 55%, respectively; P=0.04) (Figure 2). The superior outcomes with HMA+Ven were also observed when the comparison was restricted to those treated with IC (P=0.01).
Then we evaluated the impact of age specifically in pts treated with IC (stratified into <60 and ≥60 yrs) and HMA+Ven (of any age). Pts treated with HMA+Ven (93% of whom were ≥60 yrs of age) had significantly higher OS compared with pts ≥60 yrs of age who received IC (5.8m vs 4.1m, 1-yr OS 35% vs. 12%; P=0.009) (Figure 3). Although not statistically significant, there was also a trend towards superior OS with HMA+Ven compared to IC in pts <60 yrs of age who received IC (5.8m vs 5.0m, 1-yr OS 35% vs. 16%; P=0.16).
Conclusion: For pts with ts-AML and prior HMA exposure, HMA+Ven yielded significantly higher ORR rates and improved OS compared to IC/LIC, particularly in pts with non-adverse risk karyotype and in pts ≥60 yrs of age. These results suggest that HMA+Ven should be preferentially considered for pts with ts-AML and prior HMA exposure, rather than chemotherapy-based approaches. Our results also highlight the very poor outcomes of ts-AML, a poor-risk subgroup of AML for which novel, effective therapies are still needed.
Kadia: Amgen: Other: Grant/research support; AstraZeneca: Other; Cure: Speakers Bureau; BMS: Other: Grant/research support; Jazz: Consultancy; Genentech: Consultancy, Other: Grant/research support; Liberum: Consultancy; Novartis: Consultancy; Pfizer: Consultancy, Other; Pulmotech: Other; Sanofi-Aventis: Consultancy; Cellonkos: Other; Ascentage: Other; Genfleet: Other; Astellas: Other; Dalichi Sankyo: Consultancy; Aglos: Consultancy; AbbVie: Consultancy, Other: Grant/research support. Ravandi: Amgen: Honoraria, Research Funding; AstraZeneca: Honoraria; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astex: Honoraria, Research Funding; Novartis: Honoraria; Prelude: Research Funding; Taiho: Honoraria, Research Funding; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding. DiNardo: ImmuneOnc: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Agios/Servier: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Novartis: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Forma: Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding. Daver: FATE Therapeutics: Research Funding; Glycomimetics: Research Funding; Amgen: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Novartis: Consultancy; Novimmune: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Trovagene: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Pfizer: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; Hanmi: Research Funding; Trillium: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Borthakur: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; University of Texas MD Anderson Cancer Center: Current Employment; GSK: Consultancy; Protagonist: Consultancy; Ryvu: Research Funding; Astex: Research Funding; ArgenX: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Konopleva: Rafael Pharmaceuticals: Other: grant support, Research Funding; Cellectis: Other: grant support; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Sanofi: Other: grant support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; AstraZeneca: Other: grant support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; Agios: Other: grant support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Stemline Therapeutics: Research Funding; Ablynx: Other: grant support, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; KisoJi: Research Funding; Ascentage: Other: grant support, Research Funding; Calithera: Other: grant support, Research Funding; Forty Seven: Other: grant support, Research Funding. Yilmaz: Pfizer: Research Funding; Daiichi-Sankyo: Research Funding. Issa: Syndax Pharmaceuticals: Research Funding; Novartis: Consultancy, Research Funding; Kura Oncology: Consultancy, Research Funding. Kantarjian: Ipsen Pharmaceuticals: Honoraria; NOVA Research: Honoraria; Astellas Health: Honoraria; Immunogen: Research Funding; Novartis: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Aptitude Health: Honoraria; Astra Zeneca: Honoraria; KAHR Medical Ltd: Honoraria; Jazz: Research Funding; Pfizer: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; BMS: Research Funding; Ascentage: Research Funding; Amgen: Honoraria, Research Funding; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria. Short: Novartis: Honoraria; NGMBio: Consultancy; AstraZeneca: Consultancy; Jazz Pharmaceuticals: Consultancy; Astellas: Research Funding; Takeda Oncology: Consultancy, Research Funding; Amgen: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal